Differences between Adhesion and Cohesion
Contents
Comparison Article[edit]
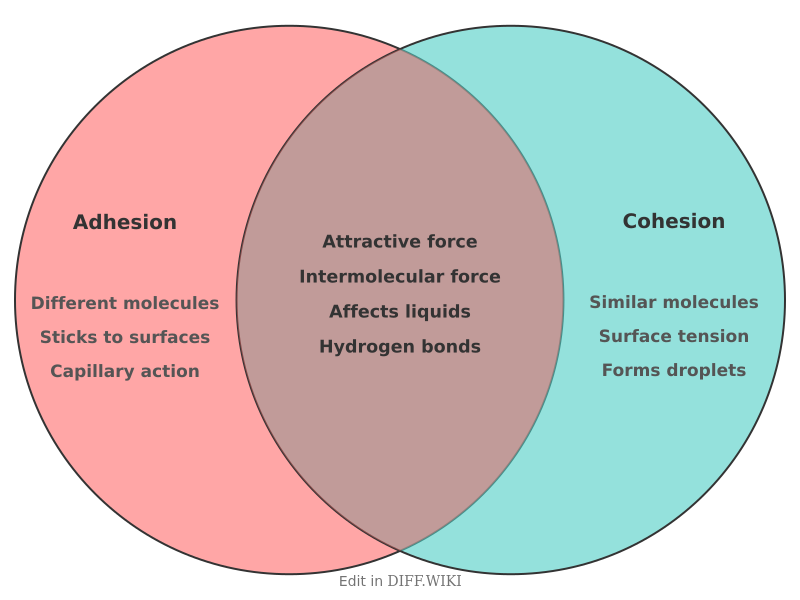
In physics and chemistry, adhesion and cohesion are attractive forces between molecules that are responsible for several observable phenomena.[1][2] Adhesion is the force of attraction between molecules of different substances.[3] Cohesion is the force of attraction between molecules of the same substance.[3] These forces are present in solids, liquids, and gases, but are most commonly observed in the behavior of liquids.[4] The balance between adhesion and cohesion determines how a liquid interacts with a solid surface.[5]
For example, when water is placed on a clean glass surface, the adhesive forces between the water molecules and the glass are stronger than the cohesive forces among the water molecules. This causes the water to spread out. Conversely, if water is placed on a waxy surface, the cohesive forces within the water are stronger than the adhesive forces between the water and the wax, causing the water to form beads or droplets.[5]
Comparison Table[edit]
| Category | Adhesion | Cohesion |
|---|---|---|
| Definition | Attraction between molecules of different substances.[1] | Attraction between molecules of the same substance.[1] |
| Interacting Particles | Unlike molecules or surfaces (e.g., water and glass). | Like molecules (e.g., two water molecules). |
| Underlying Forces | Primarily electrostatic or mechanical forces between different substances. | Primarily hydrogen bonds and van der Waals forces within a single substance. |
| Common Examples | Dewdrops clinging to a leaf; paint sticking to a wall;[1] medical tape adhering to skin.[1] | Water forming droplets;[5] insects walking on water; mercury forming beads. |
| Resulting Phenomena | Causes capillary action, wetting of surfaces, and the formation of a concave meniscus. | Causes surface tension and the formation of a convex meniscus (as with mercury).[3] |
Surface Tension[edit]
Cohesion is responsible for surface tension, which is the tendency of a liquid's surface to resist rupture when under stress. Molecules within the bulk of a liquid are pulled equally in all directions by neighboring molecules. However, molecules at the surface experience a net inward pull from the molecules below them because there are fewer molecules above the surface. This inward force minimizes the surface area, causing the surface to behave like a stretched elastic sheet. The high surface tension of water, caused by strong hydrogen bonds between its molecules, allows some insects, such as the water strider, to stay afloat on its surface.[4]
Capillary Action[edit]
Capillary action is the movement of a liquid through narrow spaces, even against gravity. This phenomenon occurs because of a combination of adhesion and cohesion. In a thin tube, such as the xylem in a plant stem, the adhesion between the water and the tube's walls pulls the water upward.[1] Cohesion ensures that the water molecules below are pulled up along with them.[1][4] The relative strengths of the adhesive and cohesive forces determine the shape of the liquid's surface in a tube, known as the meniscus. For water in a glass tube, strong adhesion creates a concave meniscus, while mercury's strong cohesion results in a convex meniscus.[3]
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "geeksforgeeks.org". Retrieved November 12, 2025.
- ↑ "libretexts.org". Retrieved November 12, 2025.
- ↑ 3.0 3.1 3.2 3.3 "ebsco.com". Retrieved November 12, 2025.
- ↑ 4.0 4.1 4.2 "byjus.com". Retrieved November 12, 2025.
- ↑ 5.0 5.1 5.2 "biolinscientific.com". Retrieved November 12, 2025.