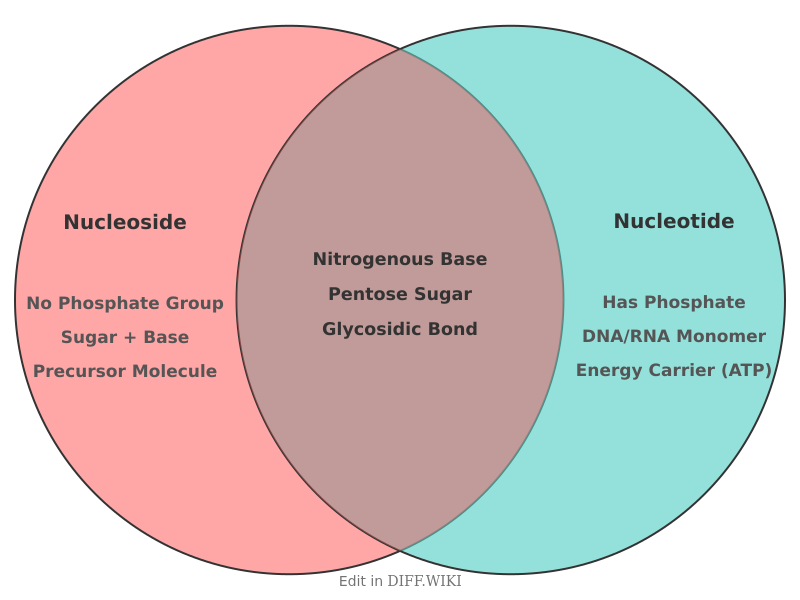
Differences between Nucleoside and Nucleotide
Nucleoside vs. Nucleotide[edit]
Nucleosides and nucleotides are fundamental organic molecules that serve as the monomeric units for nucleic acids like DNA and RNA.[1] While structurally similar, the primary distinction between them lies in their chemical composition. A nucleoside consists of a nitrogenous base covalently attached to a pentose sugar (either ribose or deoxyribose).[2] A nucleotide, in contrast, is a nucleoside that is bonded to one or more phosphate groups.[3][4]
The nitrogenous bases are classified into two main groups: purines (adenine and guanine), which have a double-ring structure, and pyrimidines (cytosine, thymine, and uracil), which have a single-ring structure.[5] The pentose sugar in RNA is ribose, whereas in DNA it is deoxyribose, which lacks a hydroxyl group at the 2' carbon position.
Nucleotides are the building blocks for the synthesis of nucleic acids.[4] Beyond their role in genetics, nucleotides are also involved in cellular energy transfer, with adenosine triphosphate (ATP) being a primary example. They also participate in cell signaling and act as cofactors in enzymatic reactions.[2] Nucleosides can be converted into nucleotides through a process called phosphorylation, catalyzed by enzymes known as kinases.[4][5] Certain nucleoside analogs are utilized as antiviral or anticancer agents.[4]
Comparison Table[edit]
| Category | Nucleoside | Nucleotide |
|---|---|---|
| Components | A nitrogenous base and a pentose sugar.[2] | A nitrogenous base, a pentose sugar, and one or more phosphate groups.[1] |
| Phosphate Group | Absent.[3] | Present.[3] |
| Primary Function | Precursor for nucleotide synthesis; role in cellular signaling. | Monomer of DNA and RNA; energy currency (e.g., ATP); cell signaling.[2] |
| Bonding | The base is linked to the sugar via a β-glycosidic bond. [2] | The nucleoside is linked to the phosphate group via a phosphoester bond. |
| Examples | Adenosine, guanosine, cytidine, uridine, thymidine. [2] | Adenosine monophosphate (AMP), Guanosine triphosphate (GTP), Deoxyadenosine monophosphate (dAMP). |
| Nomenclature Suffix | Purines end in "-osine" (e.g., adenosine), and pyrimidines end in "-idine" (e.g., cytidine). | Named by adding "monophosphate," "diphosphate," or "triphosphate" to the nucleoside name (e.g., adenosine monophosphate). |
Nomenclature[edit]
The naming conventions for nucleosides and nucleotides are systematic. For nucleosides, the suffix of the nitrogenous base's name is changed. If the base is a purine, the "-ine" ending is replaced with "-osine" (e.g., adenine becomes adenosine). If the base is a pyrimidine, the ending becomes "-idine" (e.g., cytosine becomes cytidine).
Nucleotides are named by taking the name of the corresponding nucleoside and adding the appropriate term for the number of phosphate groups attached, such as monophosphate, diphosphate, or triphosphate. For example, the nucleoside adenosine, when bonded to one phosphate group, is called adenosine monophosphate (AMP). If the sugar is deoxyribose, the prefix "deoxy-" is added to the nucleoside's name, as in deoxyadenosine.
References[edit]
- ↑ 1.0 1.1 "wikipedia.org". Retrieved November 21, 2025.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "microbenotes.com". Retrieved November 21, 2025.
- ↑ 3.0 3.1 3.2 "vedantu.com". Retrieved November 21, 2025.
- ↑ 4.0 4.1 4.2 4.3 "differencebetween.com". Retrieved November 21, 2025.
- ↑ 5.0 5.1 "abcam.co.jp". Retrieved November 21, 2025.