Differences between Purines and Pyrimidines
Contents
Differences between Purines and Pyrimidines
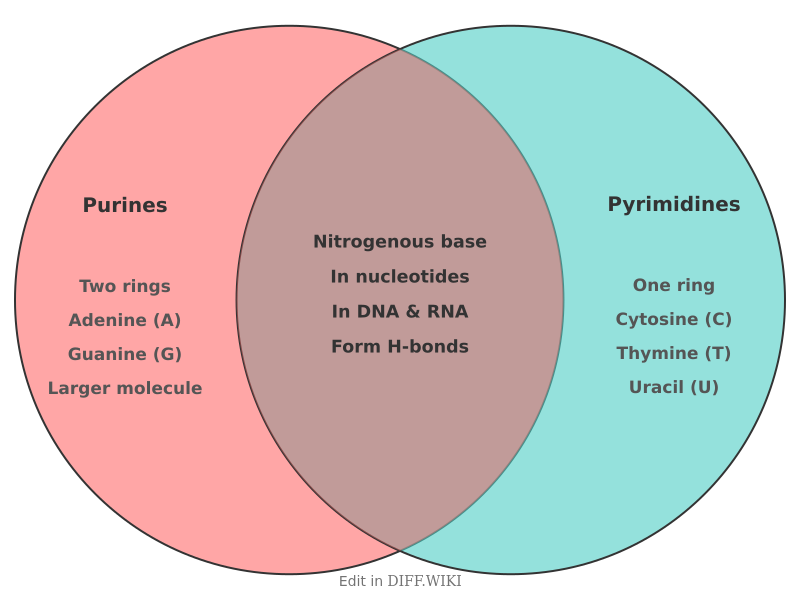
Purines and pyrimidines are nitrogenous bases, which are organic compounds that serve as the building blocks for the nucleic acids DNA and RNA.[1] While they have similar functions, their chemical structures and metabolic pathways are distinct.[2][1] The two types of bases are found in roughly equal amounts in nucleic acids.[3]
The fundamental difference between them is their structure. Purines have a double-ring structure, consisting of a six-membered ring fused to a five-membered ring.[4] Pyrimidines, in contrast, have a smaller, single-ring structure made of a single six-membered ring.[4][5] The primary purines found in both DNA and RNA are adenine and guanine. The pyrimidines are cytosine, thymine, and uracil. Cytosine is present in both DNA and RNA, while thymine is found almost exclusively in DNA, and uracil is found in RNA.[5]
Comparison Table
| Feature | Purines | Pyrimidines |
|---|---|---|
| Basic Structure | Double-ring (a pyrimidine ring fused to an imidazole ring)[3] | Single six-membered ring[3] |
| Size | Larger molecule[4] | Smaller molecule[4] |
| Examples in Nucleic Acids | Adenine (A) and Guanine (G)[2] | Cytosine (C), Thymine (T), and Uracil (U)[2] |
| Present in DNA | Adenine, Guanine | Cytosine, Thymine |
| Present in RNA | Adenine, Guanine | Cytosine, Uracil |
| Atoms in Ring Structure | Five carbon and four nitrogen atoms[3] | Four carbon and two nitrogen atoms |
| End Product of Catabolism | Uric acid[3] | Beta-amino acids, ammonia, and carbon dioxide[3] |
Base Pairing
Within the DNA double helix structure, a purine on one strand always forms hydrogen bonds with a pyrimidine on the opposing strand.[3] This pairing is specific: adenine pairs with thymine using two hydrogen bonds, and guanine pairs with cytosine using three hydrogen bonds.[2] In RNA, adenine pairs with uracil instead of thymine.[3] This complementary pairing maintains the uniform width of the DNA double helix.
Metabolism
The human body can synthesize these bases or break them down.[3] The catabolism, or breakdown, of these two types of molecules yields different end products. Purine catabolism results in the formation of uric acid, which is then excreted.[3] The breakdown of pyrimidines produces beta-amino acids, such as β-alanine and β-aminoisobutyrate, as well as ammonia and carbon dioxide. These products are water-soluble and can be reused by the body or excreted with minimal clinical impact.
References
- ↑ 1.0 1.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedref1 - ↑ 2.0 2.1 2.2 2.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedref2 - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Cite error: Invalid
<ref>tag; no text was provided for refs namedref3 - ↑ 4.0 4.1 4.2 4.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedref4 - ↑ 5.0 5.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedref5