Differences between Diffusion and Osmosis
Diffusion vs. Osmosis[edit]
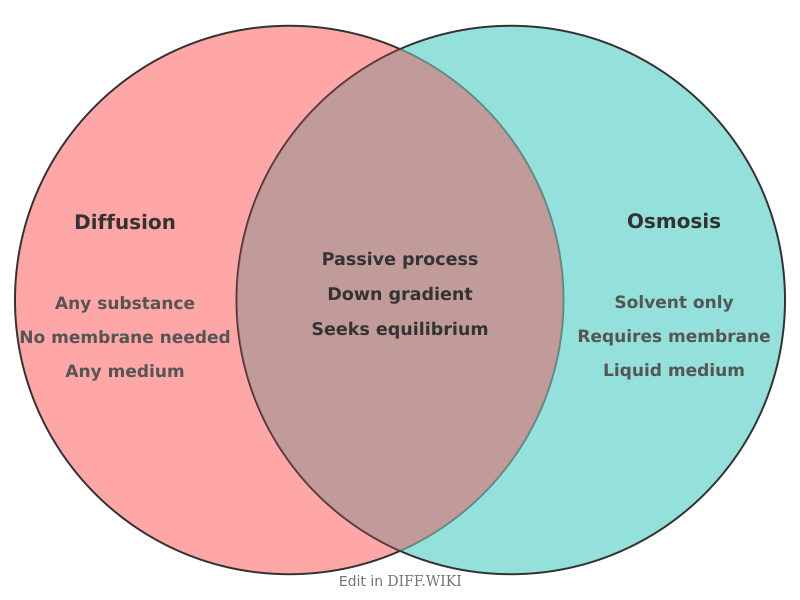
Diffusion and osmosis are two related types of passive transport, a process by which substances move across a cell membrane without the use of cellular energy.[1][2] Both processes involve the movement of molecules from an area of higher concentration to an area of lower concentration, following a concentration gradient.[3][4] The primary differences between them relate to the substance that moves, the medium, and the requirement of a semipermeable membrane.[5] Diffusion is the general process of particle movement, while osmosis describes specifically the movement of a solvent, such as water, across a membrane.
Comparison table[edit]
| Feature | Diffusion | Osmosis |
|---|---|---|
| Definition | The net movement of any particles (atoms, molecules, ions) from a region of higher concentration to a region of lower concentration.[1] | The net movement of solvent molecules (typically water) across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration. |
| Particles involved | Solute and solvent particles. | Primarily solvent molecules (e.g., water). |
| Membrane requirement | Does not require a membrane. | Requires a semipermeable membrane. |
| Medium | Can occur in any medium (solid, liquid, or gas). | Occurs only in a liquid medium. |
| Direction of flow | Down the concentration gradient of the diffusing substance until equilibrium is reached.[1] | Towards the region of higher solute concentration (or lower solvent concentration) until equilibrium is approached. |
| Biological example | Exchange of oxygen and carbon dioxide in the lungs and bloodstream.[4] | Absorption of water from the soil by plant roots. |
Biological significance[edit]
In biological systems, diffusion is essential for processes where small, nonpolar molecules need to move short distances. This includes the transport of oxygen from the alveoli in the lungs into the blood, and the movement of carbon dioxide in the opposite direction.[4] It is also the mechanism by which nutrients are transported from the blood to body tissues and waste products are removed.[4]
Osmosis is critical for the water balance within cells and organisms.[5] In plants, osmosis allows roots to absorb water from the soil and helps maintain turgor pressure, which keeps the plant upright. In animals, osmosis is involved in the function of the kidneys and the reabsorption of water in the intestines. Animal cells, which lack a rigid cell wall, can shrivel in a hypertonic solution (high solute concentration) or burst in a hypotonic solution (low solute concentration) due to osmotic water movement.
References[edit]
- ↑ 1.0 1.1 1.2 "wikipedia.org". Retrieved December 16, 2025.
- ↑ "byjus.com". Retrieved December 16, 2025.
- ↑ "thoughtco.com". Retrieved December 16, 2025.
- ↑ 4.0 4.1 4.2 4.3 "byjus.com". Retrieved December 16, 2025.
- ↑ 5.0 5.1 "ionexchangeglobal.com". Retrieved December 16, 2025.