Differences between Covalent Bonds and Ionic Bonds
Contents
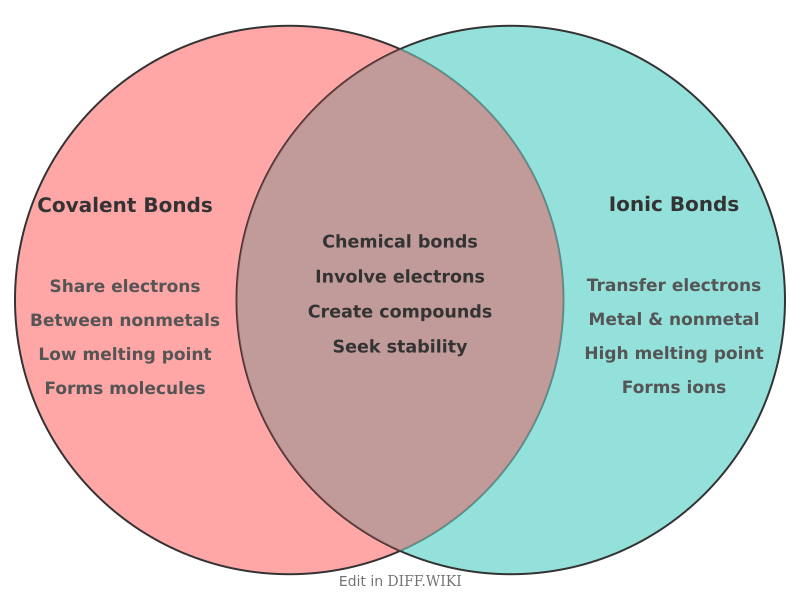
Covalent Bonds vs. Ionic Bonds
A chemical bond is a lasting attraction between atoms that allows the formation of chemical compounds.[1] The two main types of chemical bonds are covalent and ionic bonds, and the distinction between them relates to how electrons are distributed between the atoms.[2] Ionic bonds are formed from the electrostatic attraction between oppositely charged ions, a result of the complete transfer of valence electrons from one atom to another.[3][4] In contrast, covalent bonds involve the sharing of electron pairs between two atoms.[1][5]
The type of bond that forms is largely determined by the electronegativity difference between the participating atoms. A large difference in electronegativity, typically greater than 1.7, leads to the transfer of electrons and the formation of an ionic bond. When the electronegativity values are similar, atoms share electrons, resulting in a covalent bond.[5] Ionic bonds typically form between a metal and a non-metal, where the metal atom loses electrons to become a positively charged cation and the non-metal atom gains electrons to become a negatively charged anion.[2][1][3] Covalent bonds generally form between two non-metal atoms.[2]
These differences in bonding lead to significant variations in the physical properties of the resulting compounds. Ionic compounds typically form hard, brittle crystalline solids with high melting and boiling points due to the strong electrostatic forces between ions. Covalent compounds, which consist of discrete molecules with weaker intermolecular forces, are often gases, liquids, or soft solids with low melting and boiling points.
Comparison Table
| Category | Covalent Bond | Ionic Bond |
|---|---|---|
| Electron Behavior | Electrons are shared between atoms.[1][5] | Electrons are completely transferred from one atom to another.[2][4] |
| Bond Formation | Overlapping of valence orbitals between two non-metal atoms. | Electrostatic attraction between a positively charged cation and a negatively charged anion.[3][4] |
| Types of Elements | Typically forms between two non-metals.[2] | Typically forms between a metal and a non-metal.[2][3] |
| Electronegativity Difference | Small (generally less than 1.7). | Large (generally greater than 1.7). |
| Physical State at Room Temperature | Often gases, liquids, or low-melting-point solids.[5] | Usually crystalline solids. |
| Melting & Boiling Points | Generally low. | Generally high. |
| Electrical Conductivity | Poor in all states because no free-moving charged particles are present. | Conducts electricity when molten or dissolved in water, but not as a solid. |
| Solubility in Water | Variable; many are insoluble in water. | Many are soluble in water. |
Structure and Conductivity
The structure of compounds formed by these bonds differs significantly. Ionic compounds form a crystal lattice, which is a highly ordered, three-dimensional arrangement of alternating positive and negative ions. This rigid structure explains why ionic solids are not electrically conductive, as the ions are fixed in place. However, when an ionic compound is melted or dissolved in a polar solvent like water, the ions become free to move and can conduct electricity.
Covalent compounds form discrete molecules with a specific shape. The forces between these individual molecules, known as intermolecular forces, are much weaker than the electrostatic forces in an ionic lattice. Since covalent compounds consist of neutral molecules, they do not have free-moving charged particles and are therefore generally poor conductors of electricity in any state.
References
- ↑ 1.0 1.1 1.2 1.3 "allen.in". Retrieved November 14, 2025.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "thoughtco.com". Retrieved November 14, 2025.
- ↑ 3.0 3.1 3.2 3.3 "libretexts.org". Retrieved November 14, 2025.
- ↑ 4.0 4.1 4.2 "britannica.com". Retrieved November 14, 2025.
- ↑ 5.0 5.1 5.2 5.3 "wikipedia.org". Retrieved November 14, 2025.