Differences between Absorption and Adsorption
Contents
Absorption vs. Adsorption[edit]
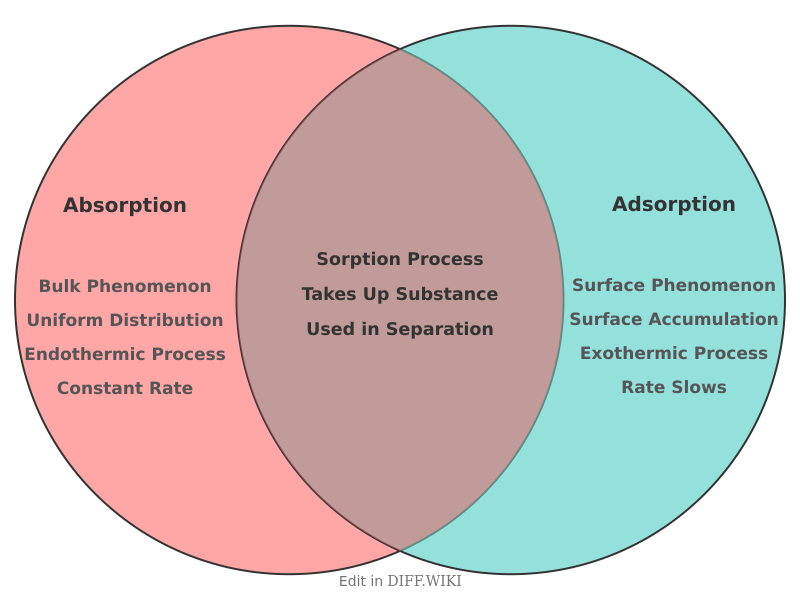
Absorption and adsorption are processes that involve the capture and transfer of substances, but they differ fundamentally in their mechanisms.[1][2] Absorption is a bulk phenomenon where a substance in one state is taken up into the volume of another material, which can be a solid or a liquid.[3][4] In contrast, adsorption is a surface phenomenon where particles adhere to the surface of a material.[5][1]
In absorption, the substance being taken up, known as the absorbate, diffuses into the absorbent material, resulting in a uniform distribution throughout the bulk of the absorbent. A common example is a sponge soaking up water; the water molecules are distributed throughout the entire sponge structure.
In adsorption, the substance that accumulates on the surface is called the adsorbate, and the material it adheres to is the adsorbent.[5] This process occurs because of unsatisfied attractive forces on the surface of the adsorbent. An example is the use of activated charcoal in filters, where impurities in water or air stick to the vast surface area of the charcoal.[2]
The distinction also extends to the thermodynamics of the processes. Absorption is typically an endothermic process, meaning it requires an input of energy. Adsorption, on the other hand, is an exothermic process that releases energy as the adsorbate molecules settle onto the surface of the adsorbent.
Comparison Table[edit]
| Category | Absorption | Adsorption |
|---|---|---|
| Phenomenon | A bulk process where a substance enters the volume of another.[1][3] | A surface process where a substance adheres to the surface of another.[5][1] |
| Mechanism | Molecules of the absorbate are distributed throughout the absorbent. | Molecules of the adsorbate accumulate on the surface of the adsorbent.[5] |
| Heat Exchange | Generally an endothermic process (absorbs heat). | Generally an exothermic process (releases heat). |
| Rate | Occurs at a relatively uniform rate. | The rate is initially fast and then slows as the surface becomes saturated. |
| Concentration | The concentration of the absorbed substance is uniform throughout the material. | The concentration of the adsorbed substance is higher on the surface than in the bulk. |
| Temperature Effect | Generally independent of temperature. | Favored at lower temperatures. |
| Example | A sponge soaking up water. | Silica gel adsorbing moisture from the air. |
Types of Interaction[edit]
Both absorption and adsorption can be categorized based on the nature of the interaction between the substances involved.
Absorption[edit]
- Physical Absorption: This is a process where the absorbed substance does not chemically react with the absorbing material. An example is oxygen dissolving in water.[3] The process relies on physical properties like solubility.[3]
- Chemical Absorption: In this type, a chemical reaction occurs between the absorbate and the absorbent.[3] An example is the removal of hydrogen sulfide from biogas streams, where it is converted into solid sulfur.[3]
Adsorption[edit]
- Physisorption (Physical Adsorption): The adhesion of the adsorbate to the adsorbent's surface is due to weak intermolecular forces, such as van der Waals forces.[3] This process is reversible.
- Chemisorption (Chemical Adsorption): This involves the formation of chemical bonds (usually covalent) between the adsorbate and the adsorbent, resulting in a stronger and often irreversible adhesion.[3]
References[edit]
- ↑ 1.0 1.1 1.2 1.3 "geeksforgeeks.org". Retrieved November 26, 2025.
- ↑ 2.0 2.1 "sciencenotes.org". Retrieved November 26, 2025.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "chromatographytoday.com". Retrieved November 26, 2025.
- ↑ "unacademy.com". Retrieved November 26, 2025.
- ↑ 5.0 5.1 5.2 5.3 "byjus.com". Retrieved November 26, 2025.