Differences between Acid and Base
Contents
Differences between acid and base[edit]
Acids and bases are chemical compounds with opposite properties.[1] When dissolved in water, an acid increases the concentration of hydrogen ions, while a base produces hydroxide ions.[2][3] Their reactions are important in industrial processes, agriculture, and biological functions.[1]
Comparison Table[edit]
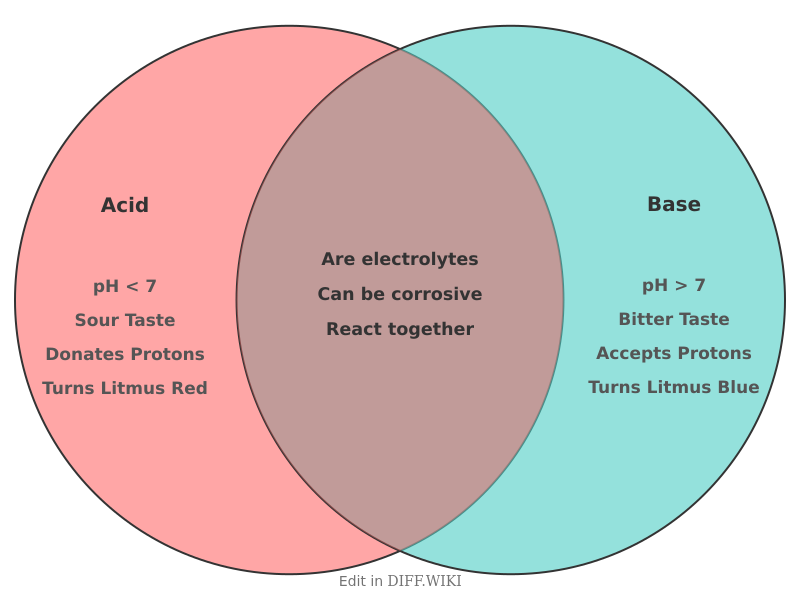
| Property | Acid | Base |
|---|---|---|
| pH | Less than 7[4][5] | Greater than 7[4][5] |
| Taste | Sour (e.g., lemon juice) | Bitter (e.g., soap) |
| Feel | Can cause a stinging sensation | Slippery or soapy to the touch[1] |
| Litmus Test | Turns blue litmus paper red | Turns red litmus paper blue |
| Reaction with Metals | Reacts with most metals to produce hydrogen gas | Usually does not react with metals, though some may react with zinc or aluminum |
| Conductivity | Conducts electricity in an aqueous solution | Conducts electricity in an aqueous solution |
| Examples | Vinegar (acetic acid), lemon juice (citric acid), hydrochloric acid (HCl) | Soap, baking soda (sodium bicarbonate), sodium hydroxide (NaOH)[2] |
Theoretical Models[edit]
Several theories define the behavior of acids and bases. Each model has expanded on the previous one, offering a broader perspective on what constitutes an acid or a base.
Arrhenius Theory[edit]
Proposed by Svante Arrhenius in 1884, this theory is the oldest of the three. It defines acids as substances that ionize in water to produce hydrogen ions (H⁺), and bases as substances that produce hydroxide ions (OH⁻) in water. A limitation of this theory is that it only applies to acid-base reactions in aqueous solutions.
Brønsted-Lowry Theory[edit]
Developed in 1923 by Johannes Nicolaus Brønsted and Thomas Martin Lowry, this theory provides a more general definition. It defines an acid as a proton (H⁺) donor and a base as a proton acceptor. This model is not restricted to aqueous solutions and can describe reactions in other solvents. When an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid.
Lewis Theory[edit]
Also proposed in 1923 by Gilbert N. Lewis, this theory is the most inclusive. It defines an acid as an electron-pair acceptor and a base as an electron-pair donor. This definition encompasses the Brønsted-Lowry theory, where the proton (H⁺) is the Lewis acid and the base that accepts it is the Lewis base. The Lewis theory can explain the acidity and basicity of substances that do not contain hydrogen, such as boron trifluoride (BF₃).
References[edit]
- ↑ 1.0 1.1 1.2 "britannica.com". Retrieved November 14, 2025.
- ↑ 2.0 2.1 "conductscience.com". Retrieved November 14, 2025.
- ↑ "solubilityofthings.com". Retrieved November 14, 2025.
- ↑ 4.0 4.1 "wikipedia.org". Retrieved November 14, 2025.
- ↑ 5.0 5.1 "usgs.gov". Retrieved November 14, 2025.