Differences between Baking Powder and Baking Soda
Contents
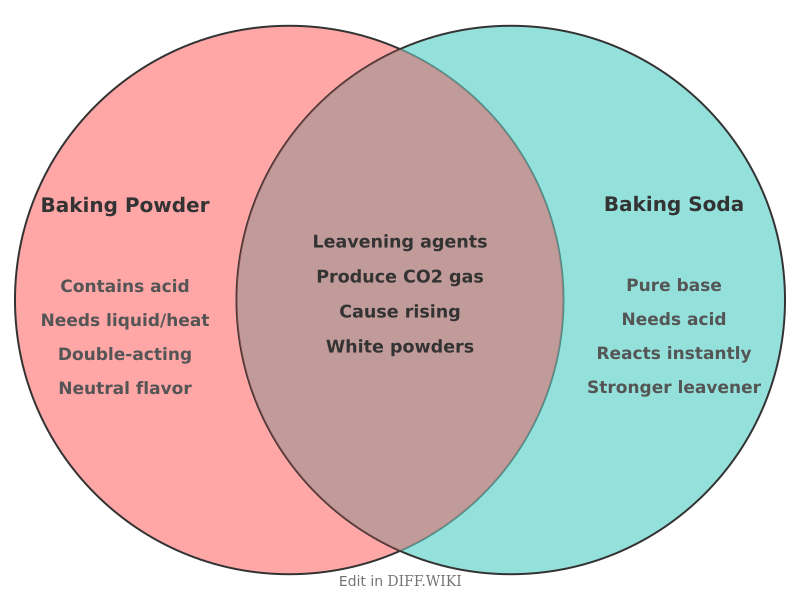
Baking Powder vs. Baking Soda[edit]
Baking powder and baking soda are both chemical leavening agents that cause batters to rise when baked.[1][2] However, they are not the same and cannot always be used interchangeably.[3] The primary active ingredient in both is sodium bicarbonate (NaHCO₃), a base. Baking[4][5] soda is purely sodium bicarbonate, while baking powder is a mixture that includes sodium bicarbonate, an acid, and a starch.
The[5] fundamental difference lies in their chemical composition and how they produce carbon dioxide gas, which leavens baked goods. Baking[4][1] soda requires an external acid source within the recipe, such as buttermilk, yogurt, lemon juice, or vinegar, to initiate the leavening reaction. In contrast, baking powder is a complete leavening system, containing both the base (sodium bicarbonate) and the necessary acid for the reaction.
[1]=== Comparison Table ===
[5]| Activation Requirement || An acidic ingredient (e.g., buttermilk, vinegar, cocoa powder) must be present in the recipe || Only[5] moisture is needed to begin the reaction; heat provides a secondary reaction| Category | Baking Soda | Baking Powder |
|---|---|---|
| Primary Component | 100% Sodium Bicarbonate (NaHCO₃) | Sodium[3] Bicarbonate, a dry acid (e.g., cream of tartar), and a starch |
| Reaction Speed | Reacts instantly upon contact with acid and moisture | Often "double-acting," with an initial reaction to moisture and a second to heat |
| Leavening Strength | Approximately three to four times stronger than baking powder | Less potent due to the inclusion of other ingredients |
| Taste Profile | Can leave a soapy or chemical taste if not fully neutralized by acid | Generally neutral in taste |
| Typical Use | Recipes that include a significant acidic ingredient | Recipes[5][1] with non-acidic ingredients like milk or Dutch-processed cocoa |
Chemical Composition and Reaction[edit]
Baking soda is a single chemical compound, sodium bicarbonate, which is a base. When[5] it comes into contact with moisture and an acid, it undergoes a chemical reaction that releases carbon dioxide gas. This immediate release of gas creates bubbles in the batter, which expand upon heating, causing the baked good to rise. Heat alone can also cause sodium bicarbonate to release carbon dioxide, but this process is slower and can result in a soapy aftertaste if an acid is not present to neutralize the alkaline sodium carbonate that is also produced.
Baking powder contains sodium bicarbonate, one or more powdered acids (like monocalcium phosphate, sodium acid pyrophosphate, or cream of tartar), and a filler like cornstarch. The cornstarch absorbs ambient moisture, preventing the acid and base from reacting prematurely. Most commercially available baking powders are double-acting. They produce a small amount of leavening when mixed with wet ingredients and a more significant amount when heated in the oven. The initial reaction occurs when the first acid (e.g., monocalcium phosphate) reacts with the sodium bicarbonate in the presence of liquid. The second, heat-activated acid (like sodium acid pyrophosphate or sodium aluminum sulfate) reacts at higher temperatures, providing a sustained rise during baking.
Culinary Applications and Substitution[edit]
The choice between baking soda and baking powder depends on the other ingredients in a recipe. If a[1] recipe contains acidic components like buttermilk, brown sugar, or citrus juice, baking soda is the appropriate leavening agent. If the recipe lacks acidic ingredients, baking powder is used since it provides its own acid. Some[5] recipes call for both to provide a balanced leavening and to ensure any acids are fully neutralized.
Substitution[1] between the two is possible but requires careful adjustment. Because baking soda is much stronger, one cannot substitute it for baking powder at a one-to-one ratio. To substitute for one teaspoon of baking soda, approximately three teaspoons of baking powder are needed. Replacing baking powder with baking soda is more complex as it requires adding an acid, such as cream of tartar, to the mixture to create the necessary chemical reaction. For every teaspoon of baking powder, one-quarter teaspoon of baking soda mixed with one-half teaspoon of cream of tartar can be used.
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "sallysbakingaddiction.com". Retrieved February 01, 2026.
- ↑ "wikipedia.org". Retrieved February 01, 2026.
- ↑ 3.0 3.1 "foodandwine.com". Retrieved February 01, 2026.
- ↑ 4.0 4.1 "mcgill.ca". Retrieved February 01, 2026.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "medicalnewstoday.com". Retrieved February 01, 2026.