Differences between Catalyst and Enzyme
Contents
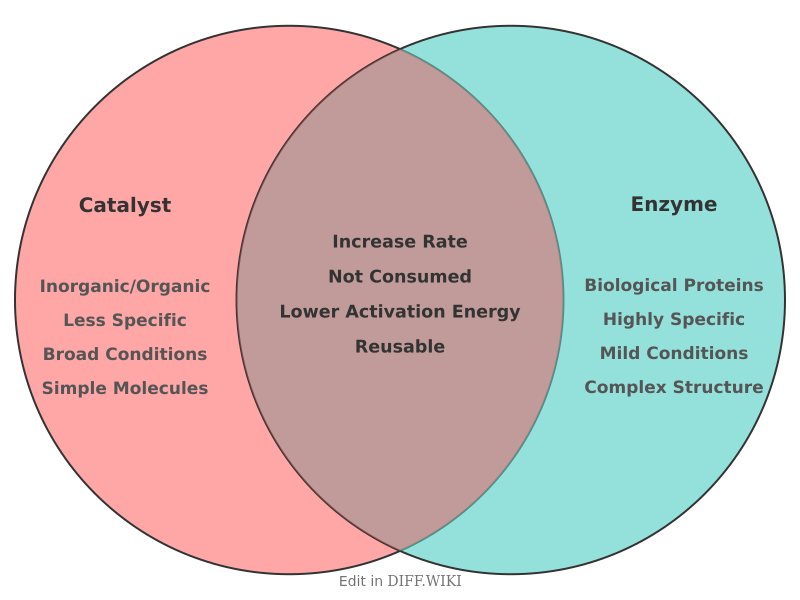
Catalyst vs. Enzyme[edit]
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process.[1][2][3] Enzymes are proteins that function as biological catalysts, speeding up biochemical reactions within living organisms.[4][5] While all enzymes are catalysts, not all catalysts are enzymes. The primary distinction lies in their composition, specificity, and the conditions under which they operate.
Comparison Table[edit]
| Category | Catalyst | Enzyme |
|---|---|---|
| Nature | Can be organic, inorganic, or metallic compounds. | Almost always a complex protein (some are RNA-based).[4] |
| Specificity | Generally have low specificity and can catalyze a variety of reactions. | Highly specific to a particular substrate and reaction. |
| Reaction Conditions | Often function under high temperature and pressure. | Function under mild physiological conditions (temperature and pH). |
| Molecular Weight | Typically have a low molecular weight. | Have a high molecular weight. |
| Regulation | Activity is generally not regulated. | Activity is tightly regulated by activators, inhibitors, and feedback mechanisms. |
| Efficiency | Generally less efficient than enzymes. | Extremely efficient, significantly increasing reaction rates. |
Composition and Structure[edit]
Catalysts are a broad category of substances that can be simple inorganic compounds, such as metals or their oxides, or organic molecules.[1] Most solid catalysts are metals or the oxides, sulfides, and halides of metallic and semimetallic elements.[1] Enzymes, in contrast, are large, complex protein molecules made up of long chains of amino acids. This complex three-dimensional structure creates a specific active site where the substrate binds and the reaction occurs.
Specificity and Mechanism[edit]
A key difference between catalysts and enzymes is their specificity. Inorganic catalysts are often not very specific and can act on a wide range of substrates and catalyze different types of reactions. Enzymes, however, are highly specific due to the unique shape of their active site, which complements the shape of a specific substrate, often compared to a lock and key mechanism. This specificity ensures that enzymes catalyze only one or a few particular reactions. Both catalysts and enzymes work by lowering the activation energy of a reaction, which is the minimum energy required for the reaction to occur, thereby increasing the reaction rate.[3] They form a temporary intermediate with the reactants, but remain unchanged after the reaction is complete.[1][2]
Reaction Conditions and Regulation[edit]
The environments in which catalysts and enzymes function effectively differ significantly. Many inorganic catalysts are robust and can withstand and operate under harsh industrial conditions, such as high temperatures and pressures. Enzymes, being proteins, are sensitive to their environment. They function optimally under the mild conditions of temperature and pH found within living organisms. Extreme temperatures or pH levels can cause the enzyme to lose its shape, a process called denaturation, which results in a loss of its catalytic activity. Furthermore, the activity of enzymes within a cell is carefully controlled by various regulatory molecules, including activators and inhibitors, which can increase or decrease their function as needed. General catalysts typically lack these sophisticated regulatory mechanisms.
References[edit]
- ↑ 1.0 1.1 1.2 1.3 "britannica.com". Retrieved December 08, 2025.
- ↑ 2.0 2.1 "wikipedia.org". Retrieved December 08, 2025.
- ↑ 3.0 3.1 "energy.gov". Retrieved December 08, 2025.
- ↑ 4.0 4.1 "britannica.com". Retrieved December 08, 2025.
- ↑ "genome.gov". Retrieved December 08, 2025.