Differences between Eszopiclone and Zolpidem
Contents
Eszopiclone vs. Zolpidem[edit]
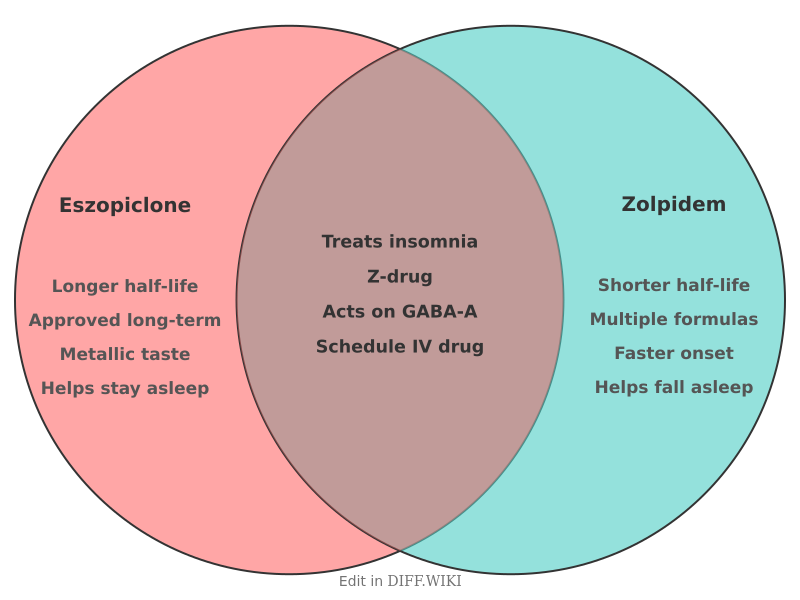
Eszopiclone and zolpidem are nonbenzodiazepine sedative-hypnotics, commonly referred to as Z-drugs, prescribed for the treatment of insomnia.[1][2] Both medications work by interacting with GABA-A receptors in the brain, which enhances the calming effect of the neurotransmitter GABA, leading to sedation.[1][2] While they share a similar mechanism of action and are both used to help people fall asleep, there are notable differences in their chemical structure, duration of action, and approved uses.[3][4] Eszopiclone is a cyclopyrrolone derivative, while zolpidem is an imidazopyridine.[1]
These medications are classified as Schedule IV controlled substances in the United States, indicating a potential for abuse and dependence.[4]
Comparison Table[edit]
| Category | Eszopiclone | Zolpidem |
|---|---|---|
| Drug Class | Cyclopyrrolone[1] | Imidazopyridine |
| Mechanism of Action | Positive allosteric modulator of GABA-A receptors[1] | Positive allosteric modulator of GABA-A receptors, with selectivity for the alpha-1 subunit[5] |
| Half-life | Approximately 6 hours[1] | Approximately 2-3 hours for immediate-release |
| Approved Uses | Treatment of insomnia, for both sleep onset and sleep maintenance. Approved for long-term use.[1] | Short-term treatment of insomnia, primarily for difficulty with sleep initiation. A lower-dose sublingual form is approved for middle-of-the-night awakenings. |
| Common Side Effects | Unpleasant or metallic taste, headache, drowsiness, dizziness, dry mouth.[1] | Drowsiness, dizziness, memory loss, headache.[4] |
| Next-Day Impairment | Risk of next-day impairment, particularly with higher doses. | Risk of next-morning impairment, which may affect activities like driving. The FDA has recommended lower doses, especially for women. |
| Formulations | Oral tablets | Oral tablets (immediate and extended-release), sublingual tablets, and an oral spray. |
Pharmacokinetics[edit]
The primary difference in the pharmacokinetic profiles of eszopiclone and zolpidem is their elimination half-life. Eszopiclone has a longer half-life of about 6 hours.[1] This longer duration of action can be beneficial for maintaining sleep throughout the night but also increases the risk of residual next-day effects such as drowsiness. In contrast, the immediate-release formulation of zolpidem has a shorter half-life of approximately 2 to 3 hours, which is often preferred for patients who have trouble falling asleep but not staying asleep.
Both drugs are metabolized in the liver, primarily by the cytochrome P450 enzyme system.[1] Specifically, CYP3A4 and CYP2E1 are involved in the metabolism of eszopiclone, while CYP3A4, CYP2C9, and CYP1A2 are the main enzymes responsible for metabolizing zolpidem.[1][5]
Clinical Efficacy and Use[edit]
Clinical studies have shown that both eszopiclone and zolpidem are effective in reducing the time it takes to fall asleep. Due to its longer half-life, eszopiclone may be more effective for sleep maintenance, helping individuals stay asleep longer. Zolpidem, particularly the immediate-release formulation, is often used to address sleep-onset insomnia.
A significant distinction in their approved use is the duration of treatment. Eszopiclone is approved by the FDA for long-term use in treating insomnia.[1] In contrast, zolpidem is generally recommended for short-term use.
Side Effects and Adverse Events[edit]
The side effect profiles of eszopiclone and zolpidem have some overlap, with both medications potentially causing drowsiness, dizziness, and next-day impairment.[1][4] A distinctive and common side effect of eszopiclone is an unpleasant or metallic taste in the mouth.[1] Zolpidem has been associated with a risk of complex sleep behaviors, such as sleepwalking or sleep-driving, which has led to an FDA boxed warning for this class of drugs. Both medications can cause serious allergic reactions, including angioedema.[1]
References[edit]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "wikipedia.org". Retrieved January 23, 2026.
- ↑ 2.0 2.1 "webmd.com". Retrieved January 23, 2026.
- ↑ "wikipedia.org". Retrieved January 23, 2026.
- ↑ 4.0 4.1 4.2 4.3 "singlecare.com". Retrieved January 23, 2026.
- ↑ 5.0 5.1 "drugs.com". Retrieved January 23, 2026.