Differences between Hard Water and Soft Water
Contents
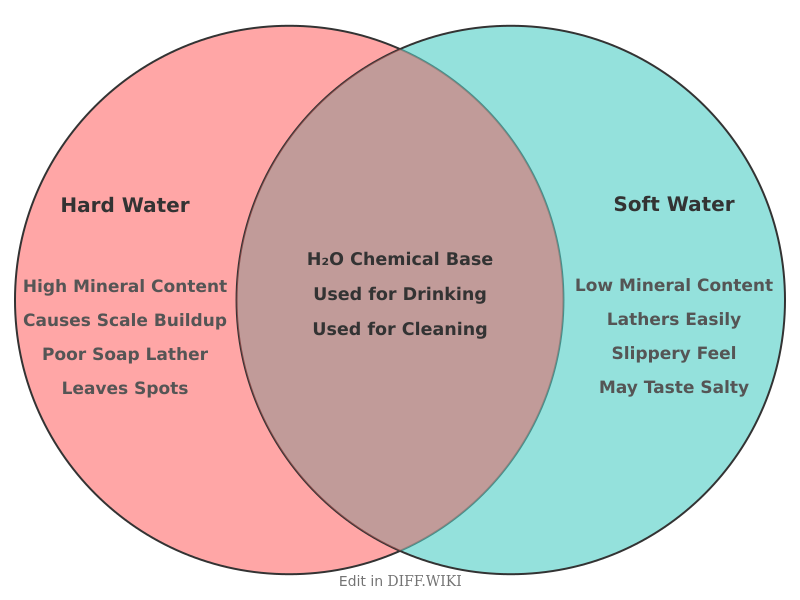
Hard Water vs. Soft Water[edit]
Water hardness is determined by the concentration of dissolved minerals, primarily calcium and magnesium.[1][2] Hard water has a high mineral content, which it acquires as it percolates through deposits of limestone, chalk, or gypsum.[3] Soft water has a low concentration of these minerals.[4] While the mineral content in hard water is generally not harmful to health, the differences between hard and soft water are noticeable in daily use, from bathing to the longevity of plumbing and appliances.[1][5]
Comparison Table[edit]
[1]| Interaction with Soap[3]| Effects on Skin and Hair| Category | Hard Water | Soft Water |
|---|---|---|
| Mineral Content | High concentration of dissolved minerals, mainly calcium (CaCO₃) and magnesium (MgCO₃). | Low concentration of dissolved minerals; may have a higher sodium ion concentration after softening. |
| Reacts with soap to form soap scum, reducing lathering ability. | Lathers[1] easily with soap and detergents without forming scum. | |
| Residue and Scale | Leaves behind hard mineral deposits (limescale) on fixtures, dishes, and inside pipes and appliances. | Leaves[4][3] little to no mineral residue or scale. |
| Effects on Plumbing | Mineral buildup can clog pipes, reduce water flow, and lead to corrosion. | Does not cause mineral scale buildup in plumbing systems. |
| Can contribute to dryness, irritation, and clogged pores by leaving a residue on skin and hair. | Rinses[1] cleanly without leaving mineral residue, which can feel slick to those accustomed to hard water. | |
| Appliance Efficiency | Scale buildup on heating elements reduces the efficiency and lifespan of water heaters and other appliances. | Allows appliances to operate more efficiently without scale accumulation. |
| Measurement | Measured in grains per gallon (GPG) or parts per million (ppm). Water over 7 GPG or 121 ppm is typically classified as hard. | Generally classified as water with less than 1 GPG or under 60 ppm of hardness minerals. |
Effects of hard water[edit]
The primary disadvantage of hard water is the formation of limescale, a hard, chalky deposit composed mainly of calcium carbonate. These[3] deposits can accumulate inside pipes, restricting water flow and reducing water pressure. In appliances like water heaters, dishwashers, and kettles, limescale builds up on heating elements, making them less efficient and shortening their lifespan.
When used for cleaning, the minerals in hard water react with soap to create an insoluble film known as soap scum, which can leave streaks on dishes and a residue on skin and hair. This[1] reaction also reduces the effectiveness of soaps and detergents, requiring more product to achieve a lather. For individuals with sensitive skin or conditions like eczema, hard water can sometimes worsen dryness and irritation.
[1]= Water softening =[edit]
Water softening is the process of removing the minerals that cause water hardness, primarily calcium and magnesium ions. The most common method for residential water softening is an ion-exchange water softener. This system works by passing hard water through a tank containing resin beads that are charged with sodium ions. The calcium and magnesium ions in the water are attracted to the resin and are "exchanged" for sodium ions, resulting in softened water. Periodically, the resin beads must be regenerated by flushing them with a concentrated salt (brine) solution, which washes away the accumulated calcium and magnesium and recharges the beads with sodium.
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "healthline.com". Retrieved February 02, 2026.
- ↑ "homewater.com". Retrieved February 02, 2026.
- ↑ 3.0 3.1 3.2 3.3 "wikipedia.org". Retrieved February 02, 2026.
- ↑ 4.0 4.1 "culligan.com". Retrieved February 02, 2026.
- ↑ "kinetico.com". Retrieved February 02, 2026.