Differences between Heat and Temperature
Contents
Comparison Article[edit]
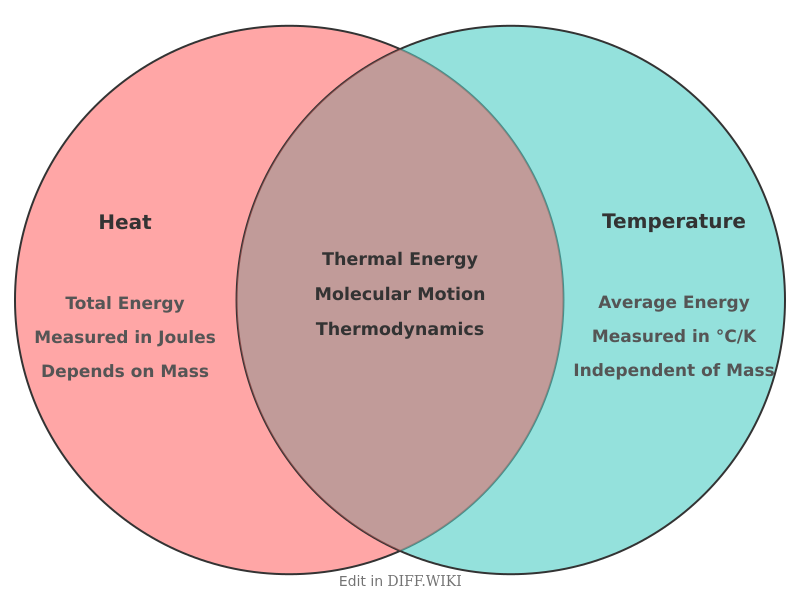
In thermodynamics, **heat** and **temperature** are closely related but distinct concepts. Temperature is a measure of the average kinetic energy of the particles within a substance, indicating how hot or cold it is.[1][2][3] Heat, in contrast, is the transfer of thermal energy between objects due to a temperature difference. This energy always flows from a hotter body to a colder one until thermal equilibrium is reached.[4][5] While the two are often used interchangeably in common language, they represent different physical quantities.[4]
Comparison table[edit]
| Category | Heat | Temperature |
|---|---|---|
| Definition | The energy transferred from one body to another as a result of a difference in temperature. | A measure of the average kinetic energy of the atoms or molecules in a system.[1] |
| SI Unit | joule (J) | kelvin (K)[1] |
| Common Symbols | Q | T |
| Measuring Instrument | Calorimeter | Thermometer |
| Physical Quantity | A measure of the total energy (kinetic and potential) of all particles in an object. It is an extensive property, meaning it depends on the mass of the substance.[2] | A measure of the average kinetic energy of particles. It is an intensive property, meaning it does not depend on the amount of substance. |
| Mechanism | Energy in transit; it flows between systems.[5] | A property of a single system in thermal equilibrium.[1] |
Measurement[edit]
Temperature is the quantity measured by a thermometer. Common temperature scales include Celsius (°C), Fahrenheit (°F), and the Kelvin (K) scale, with Kelvin being the base unit in the International System of Units (SI) used for scientific purposes.
Heat,[1] as a form of energy transfer, is measured in joules (J) in the SI system. The amount of heat transferred during a process is typically measured using a device called a calorimeter, which observes the effect of the energy transfer on a substance, such as a change in its temperature.
Relationship and distinction[edit]
The key distinction is that temperature describes the state of a system, while heat describes an energy transfer process. For[2] example, a large pot of lukewarm water can have more total thermal energy (and thus be able to transfer more heat) than a small cup of very hot water, even though the cup of water has a much higher temperature. This[4] is because the pot contains a vastly greater number of water molecules, and heat is related to the total energy of all its molecules.
The transfer of heat to a substance will typically cause its temperature to rise as the kinetic energy of its constituent particles increases. Similarly,[4] when a substance loses heat, its temperature usually decreases. However,[5] a substance can absorb or release heat without a change in temperature during a phase change, such as ice melting into water at 0 °C. In this case, the energy being transferred (latent heat) is used to change the potential energy of the molecules as they rearrange into a new state.
References[edit]
- ↑ 1.0 1.1 1.2 1.3 1.4 "wikipedia.org". Retrieved January 25, 2026.
- ↑ 2.0 2.1 2.2 "study.com". Retrieved January 25, 2026.
- ↑ "britannica.com". Retrieved January 25, 2026.
- ↑ 4.0 4.1 4.2 4.3 "wikipedia.org". Retrieved January 25, 2026.
- ↑ 5.0 5.1 5.2 "britannica.com". Retrieved January 25, 2026.