Differences between Nuclear Fission and Nuclear Fusion
Contents
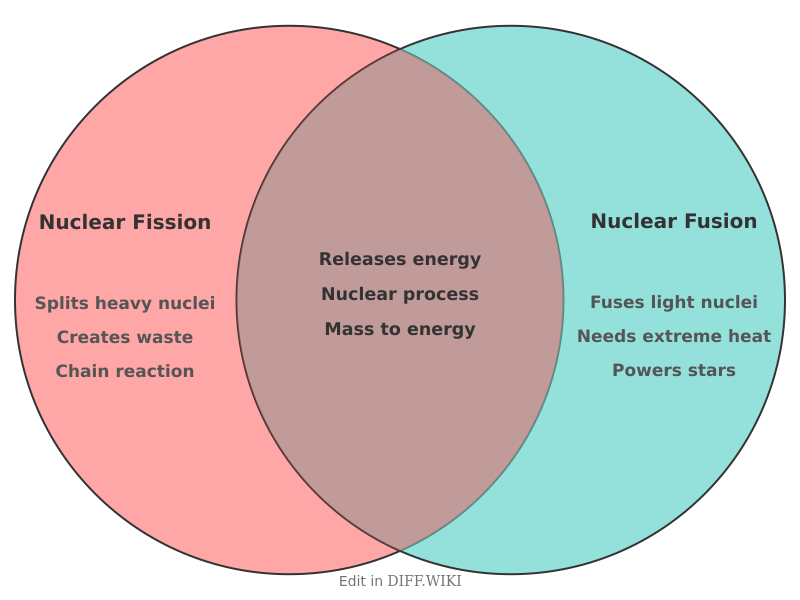
Nuclear Fission vs. Nuclear Fusion[edit]
Nuclear fission and nuclear fusion are two distinct types of nuclear reactions that release large quantities of energy.[1] Fission is the process of splitting a heavy, unstable atomic nucleus into two smaller ones.[2] Conversely, nuclear fusion is the process where two light atomic nuclei combine to form a single heavier nucleus.[3] While both processes are used in energy production, their underlying principles, applications, and byproducts differ significantly.
Comparison Table[edit]
| Category | Nuclear Fission | Nuclear Fusion |
|---|---|---|
| Process | Splitting a heavy nucleus into lighter nuclei.[2] | Combining two light nuclei to form a heavier nucleus.[2] |
| Fuel | Typically heavy elements like uranium-235 and plutonium-239.[4][5] | Typically light elements like deuterium and tritium (isotopes of hydrogen).[2][3] |
| Energy Release | Releases a large amount of energy, but less than fusion on a per-mass basis. | Releases several times more energy per kilogram of fuel than fission. |
| Natural Occurrence | Does not typically occur naturally, though some radioactive elements undergo spontaneous fission.[5] | Occurs in stars, including the Sun.[4] |
| Byproducts | Produces long-lived radioactive waste. | Primarily produces helium, an inert gas, with some short-lived radioactive material like tritium being produced and consumed within the reactor. |
| Current Applications | Used in nuclear power plants for electricity generation and in nuclear weapons.[5] | Primarily experimental, with research focused on developing fusion reactors for power generation. It is also used in thermonuclear weapons.[2] |
| Control | The chain reaction can be controlled and sustained in nuclear reactors.[4][2] | Reactions are difficult to sustain due to the extreme temperature and pressure required.[4][3] |
Nuclear Fission[edit]
Fission reactions are initiated by bombarding a heavy nucleus, such as uranium-235, with a neutron. This causes the nucleus to become unstable and split into smaller nuclei, known as fission products, while also releasing additional neutrons and a significant amount of energy.[2] If these released neutrons strike other uranium-235 nuclei, they can trigger a self-sustaining chain reaction. This controlled chain reaction is the principle behind nuclear power reactors, which harness the heat produced to generate electricity.[4] Fission is a mature technology used for commercial power generation and has applications in medicine and research.[3] A primary concern with fission is the production of radioactive waste, some of which remains hazardous for thousands of years.
Nuclear Fusion[edit]
Fusion reactions involve the merging of light atomic nuclei, such as the hydrogen isotopes deuterium and tritium, under conditions of extreme temperature and pressure.[3] This process, which powers the sun and other stars, results in the formation of a heavier nucleus, like helium, and the release of a substantial amount of energy.[4] Fusion is considered a potentially cleaner and more sustainable energy source than fission because its fuel is abundant and it does not produce long-lived radioactive waste.[2] However, achieving and maintaining the necessary conditions for a sustained fusion reaction on Earth presents significant technological challenges. Research into controlled fusion is ongoing, with the goal of developing commercially viable fusion power plants in the future.[2]
References[edit]
- ↑ "libretexts.org". Retrieved November 15, 2025.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "orano.group". Retrieved November 15, 2025.
- ↑ 3.0 3.1 3.2 3.3 3.4 "energy.gov". Retrieved November 15, 2025.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "wikipedia.org". Retrieved November 15, 2025.
- ↑ 5.0 5.1 5.2 "twi-global.com". Retrieved November 15, 2025.