Differences between Prilosec and Zantac
Contents
Prilosec vs. Zantac[edit]
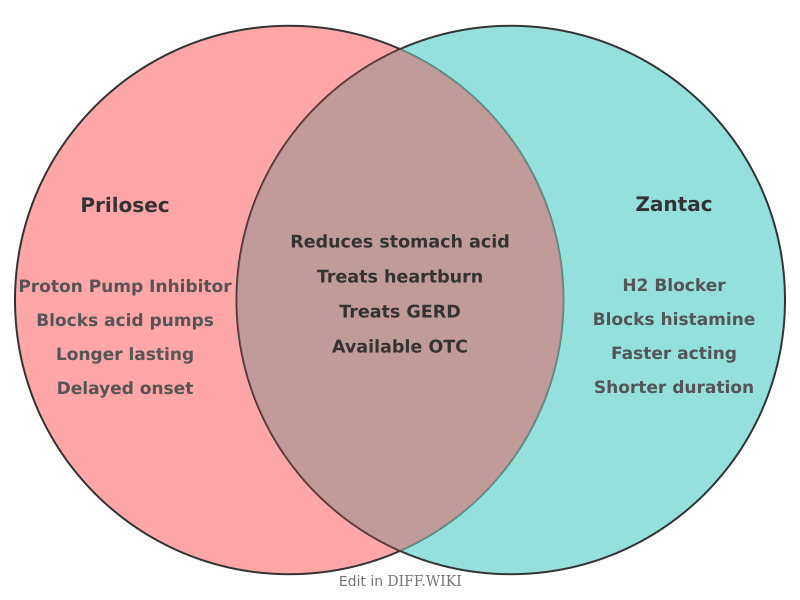
Prilosec and Zantac are brand names for medications used to reduce stomach acid.[1] While both treat similar conditions, such as heartburn and gastroesophageal reflux disease (GERD), they belong to different drug classes and function in distinct ways.[1] Prilosec's active ingredient is omeprazole, a proton-pump inhibitor (PPI).[2][3] Zantac historically contained ranitidine, a histamine H2-receptor antagonist (H2 blocker).[4] However, products marketed as Zantac 360 now contain famotidine, another H2 blocker.[5]
In 2020, the U.S. Food and Drug Administration (FDA) requested the withdrawal of all ranitidine products from the market. This action was taken due to the discovery that the impurity N-nitrosodimethylamine (NDMA), a probable human carcinogen, could increase in ranitidine products over time and when stored at higher than room temperatures.
Comparison Table[edit]
| Category | Prilosec | Zantac (ranitidine) |
|---|---|---|
| Active Ingredient | Omeprazole | Ranitidine[4] |
| Drug Class | Proton-pump inhibitor (PPI)[2] | H2-receptor antagonist (H2 blocker)[4] |
| Mechanism of Action | Blocks the enzyme in the stomach wall that produces acid.[5] | Blocks the action of histamine on stomach cells, reducing acid production.[5] |
| Primary Use | Treatment of frequent heartburn, GERD, stomach ulcers, and erosive esophagitis.[2] | Previously used for heartburn, GERD, and stomach ulcers. |
| Market Status | Available over-the-counter (OTC) and by prescription.[2] | Withdrawn from the market in 2020 due to NDMA contamination. |
Mechanism of Action[edit]
Prilosec (omeprazole) is a proton-pump inhibitor.[2] It works by irreversibly blocking an enzyme system on the surface of gastric parietal cells, which are responsible for secreting stomach acid. This action inhibits the final step in acid production, reducing both basal and stimulated acid secretion.
The original Zantac (ranitidine) was an H2 blocker. It functioned by competing with histamine for H2 receptors on the stomach's parietal cells. By blocking these receptors, it reduced the production of stomach acid.
Market Withdrawal of Zantac (ranitidine)[edit]
The withdrawal of ranitidine from the U.S. market began with voluntary recalls in late 2019 after an independent pharmacy detected NDMA in the medication. The FDA's subsequent investigation confirmed that NDMA levels could increase to unacceptable levels under normal storage conditions, particularly at higher temperatures. This led to the agency's request for the removal of all ranitidine products in April 2020. The new product, Zantac 360, uses famotidine as its active ingredient, which is another type of H2 blocker not found to have the same NDMA issue.[4]
References[edit]
- ↑ 1.0 1.1 "singlecare.com". Retrieved January 04, 2026.
- ↑ 2.0 2.1 2.2 2.3 2.4 "singlecare.com". Retrieved January 04, 2026.
- ↑ "rxlist.com". Retrieved January 04, 2026.
- ↑ 4.0 4.1 4.2 4.3 "wisnerbaum.com". Retrieved January 04, 2026.
- ↑ 5.0 5.1 5.2 "medicinenet.com". Retrieved January 04, 2026.